Making Proteins with Bugs for a More Sustainable World
Matt and Jalene Anderson-Baron on using machines made by nature to create a better world.
Their vision is a future where embracing the full human experience –like eating meat – does not compromise the health and viability of humanity and the planet. A future where innovative bioproducts feed, heal, and fuel the world.
You may have heard Future Fields’ origin story before. It all started with one fateful conversation in line at a University of Alberta Tim Hortons in 2018. While waiting to order, our current co-founders, Matt and Jalene Anderson-Baron, were discussing how to grow their cell-based chicken nugget project without the commonly used component, fetal bovine serum (FBS). They wanted an alternative that was better for the planet, and didn’t break the bank.
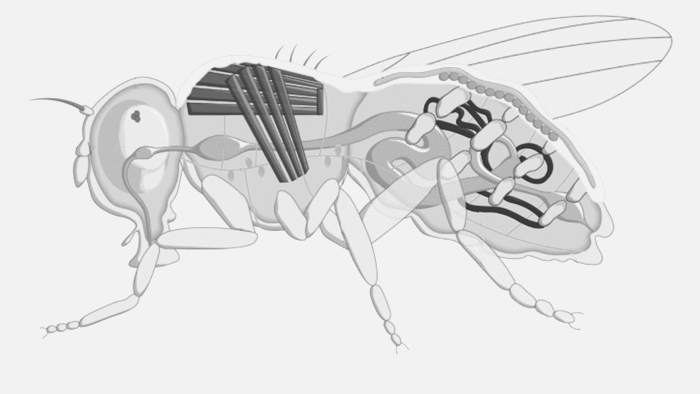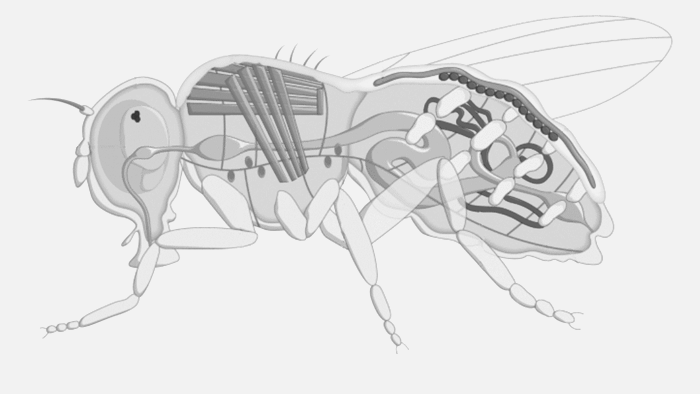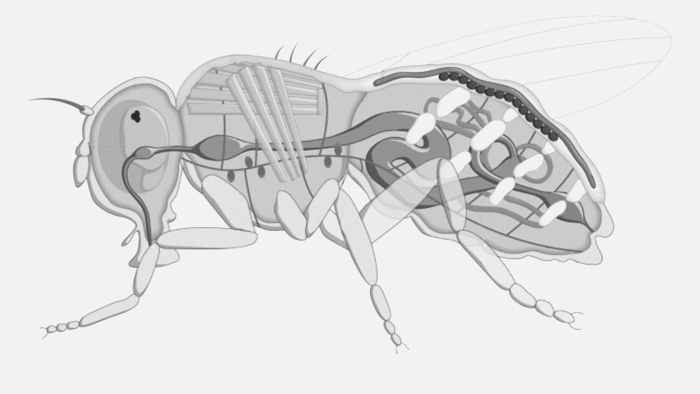
Jalene wondered if the common fruit fly, Matt’s focus of PhD studies, could offer some answers.
COMPANY PROFILE: FUTURE FIELDS
Serum-free growth factors for the sustainable scientist.
Creating custom growth media solutions for the cellular agriculture industry - cost effective, scalable, and optimized for the future of food.
FOUNDERS:
→ Matt Anderson-Baron
→ Jalene Anderson-Baron
LAST FUNDING STAGE:
Seed
VECTOR:
Biological Machines

2
Growth Factors

31
Team Members

14M
Funding to Date

Matt and Jalene
FOUNDERS
Jalene and Matt Anderson-Baron
It takes a lot of infrastructure, and money, to build the giant bioreactors experts say are needed to mass produce raw materials to make things like medicine, vaccines and cultivated meat.
Matt Anderson-Baron, co-founder and CEO of Canada-based Future Fields, told TechCrunch that 10 billion liters of bioreactor capacity will be needed by 2030, yet only 61 million liters of it exists today.
In addition, the recombinant protein, which is the output of the bioreactors, accounts for the majority of the costs associated with producing cultivated meat and is partly why that sector has not been able to reach price parity with traditional meat.
Future Fields thinks it has come up with a more cost-effective and sustainable way to do this through its EntoEngine, an approach that uses fruit flies — not giant steel tanks — for recombinant protein production.
Originally appeared in TechCrunch Future Fields is turning fruit flies into bioreactors
Jalene and Matt introduce the EntoEngine.
"We started this company because we knew that the cost of growth factors and recombinant proteins was wildly too expensive."
-Matt
“Traditionally, growth factors and recombinant proteins are produced in microbial systems grown in big stainless steel tanks, so they’re competing for the same infrastructure,” Anderson-Baron said. “Where we come in is we replace the bioreactor with an insect. We’re genetically engineering them — insects that can be grown in simple plastic containers — and it is very, very scalable, cost-effective and, importantly, circumvents the challenges associated with that infrastructure and frees that supply chain up for the people who actually need it or not, without creating more demand for it.”
Anderson-Baron, his wife, Jalene Anderson-Baron, and co-founder Lejjy Gafour have been working on this problem for a while and in early 2022 announced $11.2 million in seed extension funding that enables the company to build a production facility to launch its first products outside of cultivated meat: research, cell therapies and biopharmaceuticals, as well as scale its team.
Syndicate partners who joined Bee Partners in the round include: Toyota Ventures, Builders VC, AgFunder, Amplify Capital, Milad Alucozai of BoxOne Ventures, Green Circle Foodtech, Siddhi Capital and Climate Capital.

Matt 
Delve deeper into the world of Biological Machines through our other inspiring articles or discover why we're also passionate about Human Machine Interaction and Machine to Machine Learning.
DIG DEEPER
Related News & Insights

Bee.
Bee Partners
50 Osgood Place
Suite 220A
San Francisco, CA 94133
LinkedIn: Bee Partners
Twitter: @BeePartners
© Bee Partners 2024